Research Article
Beata Seremak1 , Lidia Felska-Błaszczyk2, Doris Rubinowska1, Aleksandra Wojciechowska1
1Department of Animal Reproducion Biotechnology and Environmental Hygiene, West Pomeranian University of Technology, Szczecin, Janickiego 29, 71-270 Szczecin, Poland
2Laboratory of Animal Anatomy, Faculty of Biotechnology and Animal Husbandry, West Pomeranian University of Technology, Szczecin, Doktora Judyma 14, 71-466 Szczecin, Poland
Abstract. The aim of the study was to describe parturitions in eight Pearl mink females. The video material, recorded inside each of the eight nests, was analysed using the Behaview software package. Duration of labour was determined, as well as the number of times female left the nest during parturition, the mean time of female’s absence from the nest, and time intervals between births of individual kits. The results show that the average duration of parturition was 3 hours and 23 min, during which a female left the nest 8 times on average, remaining outside for an average of about 18 minutes. Time intervals between individual kit births ranged from 16 to 52 minutes.
Keywords: behaviour, mink, parturition
The mink belongs to the order Carnivora, family Mustelidae, genus Mustela. The genus includes the European (Mustela lutreola) and American mink (Mustela vison or Neovison vison). Due to the desirable fur quality, the mink has been an object of cage farming. It belongs to monoestrous animals, in which the gonadotropin activity of the pituitary gland largely depends on the changing daylight period [Vidal et al. 1997Vidal, S., Lombardero, M., Moya, L. (1997). Ultrastructural and immunocytochemical studies of prolactin-secreting cells in adenohypophysis of the mink (Mustela vison). Gen. Comp. Endocrinol., 107, 311–321. https://doi.org/10.1006/gcen.1997.6926., Douglas et al. 1998Douglas, D.A., Houde, A., Song, J.H., Farookhi, R., Cancannon, P.W., Murphy, B.D. (1998). Luteotropic hormone receptors in the ovary of the mink (Mustela vison) during delayed implantation and early-postimplantation gestation. Biol. Reprod., 59(3), 571–578. https://doi.org/10.1095/biolreprod59.3.571., Vidal et al. 1999Vidal, S., Maryllera, M., Roman, A., Moya, L. (1999). Changes in estrogen receptor expression and cell activity of lactotropes in female mink (Mustela vison) pituitary in response to variations in the gonadal steroid environment. Gen. Comp. Endocrinol., 114(3), 365–377. https://doi.org/10.1006/gcen.1999.7280.] which, according to Amstislavsky and Ternovskaya [2000Amstislavsky, S.A., Ternovskaya, Y. (2000). Reproduction in mustelids. Anim. Reprod. Sci., 60–61, 571–581. https://doi.org/10.1016/s0378-4320(00)00126-3.], defines the onset of the breeding season, time of implantation and embryo development duration. Mating seasons in Poland start early March, which is associated with an extending light phase of day. The oestrus continues over a period of 7 to 20 days [Socha and Markiewicz 2002Socha, S., Markiewicz, D. (2002). Effect of mating and whelping dates on the number of pups in mink. [Online] EJPAU Anim. Husban., 5 (2).] and involves fixed, consecutive cycles of egg cell maturation and ovulation. The cycle repeats every 6–8 days [Lagerkvist et al. 1992Lagerkvist, G., Einarsson, E.J., Forsberg, M., Gustafsson, H. (1992). Profiles of oestradiol-17β and progesterone and follicular development during the reproductive season in mink (Mustela vison). J. Reprod. Fertil., 94, 11–21. https://doi.org/10.1530/jrf.0.0940011., Wehrenberg et al. 1992Wehrenberg, W.B., Kurt, K.J., Hutz, R.J. (1992). Effects of equine chorionic gonadotropin on reproductive performance in anoestrous mink. J. Anim. Sci., 70(2), 499–502. https://doi.org/10.2527/1992.702499x.], and there may be up to four of them in the season. This cyclic maturation and ovulation is linked with the libido. Reproduction performance is the key factor of a profitable farm production. The breeding season, however, represents a challenge for the farmer, as the success strongly depends on both genetics and environment.
Achieving high parameters in reproduction performance is restricted due to the complex reproduction physiology of the mink and a high offspring mortality rate during nursing. It is apparent form the literature of the subject that the research on mink is mostly focused on the most important issues of production efficiency, such as reproduction and selection for coat quality [Kondo and Nishiumi 1991Kondo, K., Nishiumi, T. (1991). The pelage development in young mink (Mustela vison). J. Fac. Agric., 64 (4), 247–255., Seremak et al. 2013Seremak, B., Felska-Błaszczyk, L., Dworecka, M., Dziadosz-Styś, M., Lasota, B. (2013). Analysis of litter sizes at birth and at 7 days of nursing in mink (Neovison vison) of Black Velvet, Hedlund White, and Silverblue color types. Acta Sci. Pol., Zootech., 12 (2), 39–48.]; however, little information on the behaviour of the species has been published so far. This is an impulse for a more in-depth analysis of the behavioural patterns of the mink managed in captivity. Particularly interesting and poorly described seem to be the reproductive behaviour during the whelping. Analysis of behaviour will allow breeders to adjust the conditions on the farm to better meet the animals' needs, both improving the welfare and contributing to a better economic performance. Behavioral study aimed at an analysis of parturitions were taken in American mink females, Pearl colour type, managed under farm conditions. The observations were primarily focused on labour duration, time intervals between births of individual kits, and the time spent by females outside the nest.
Observations took place on a mink farm located in the Westpomeranian Voivodship, Poland. The objects were Pearl mink females. Infrared cameras were placed in 8 nests and the recordings were performed on a continuous basis. The video material, showing the births, was next subjected to analysis. Animals were fed a standard semi-liquid feed, according to commonly accepted rules. During the experiment, the animals remained under the same environmental conditions – open sheds, one-storey serial cages. All nests were bedded with wood shavings, the females had access to straw placed on a hinged grid over the nests.
The data were gathered in a serial recording method, which consists in recording a range of behaviours occurring in a given group within a given time period. It enables observations of the actions of interest within a group, or a set of behaviours occurring in a single individual. The methods allows recording the frequency, duration, sequence of actions, as well as interactions between the objects.
The video material with the recorded birth processes was analysed using the Behaview package. The software was used to analyse the behaviours within a selected period by marking the beginning and end of the particular action, as well as the number of its replications. The analysis of a whelping process involved its duration, time intervals between individual kits’ births, numbers of cases when the female left the nest, as well as the total time spent outside the nest. The moment of the first birth marked the beginning, and the last birth in the litter was marked as the completion of the labour process.
The observations allowed to determine the duration of labour, intervals between individual births, number of times the female left the nest, and the total time spent outside the nest. Labour durations ranged from 1 hour and 3 minutes to 4 hours and 32 minutes. Females left their nests from only once (the most motionless female) to 22 times (the most restless female). After leaving the nest, females spent from 3 to 43 minutes some other place in the cage. The females also differed in fertility, the litters ranging from 5 to 12 young.
Experimental females number 2 and 7 were characteristic for the smallest litters, 5 kits each. Despite the equal numbers of kits, their birth ran differently. Labour duration in the 2 female was 3 hours and 30 minutes, whereas in the 7 female it was 1 hour and 3 minutes. There were considerable differences between both females in time intervals between individual kits’ births (Fig. 1). Both females seldom left their nests during labour, number 2 - 4 times, number 7 only once (Fig. 2). The total time spent outside the nest was, respectively, about 8 and about 5 minutes (Fig. 3).
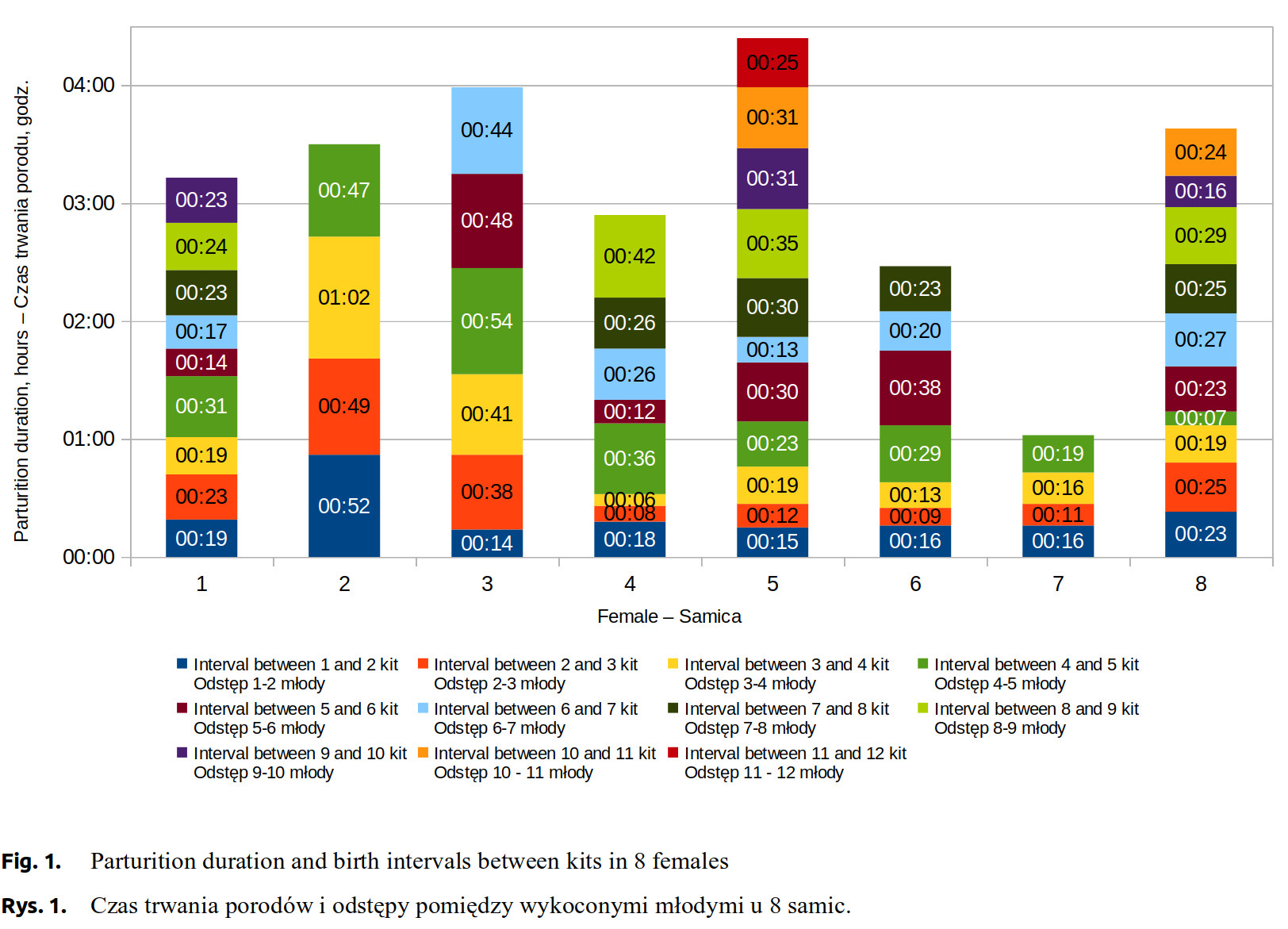
Females number 3, 4 and 6 gave birth to, respectively, 7, 9 and 8 kits. Labour duration in the female number 3 was 4 hours and 2 minutes. The interval between births of the first and the second kit was about 14 minutes and was relatively shorter from the subsequent intervals, which lasted on average 45 minutes (Fig. 1). A female left the nest 3 times, with a total time spent outside nest about 3 minutes (Fig. 2 and Fig. 3). Females number 4 and 6 exhibited similar labour characteristics. Their total labour durations were, respectively, 2 hours and 58 minutes and 2 hours and 30 minutes. In both females, the birth intervals between the kits were relatively short, until the birth of the fourth kit, which was followed by its extension (Fig. 1). The female number 4 was much more restless, left the nest 22 times, whereas the female number 6 left only 3 times. By analogy, total time spent outside the nest was higher for the female number 4, about 41 minutes, while for the female number 6 only about 13 minutes (Fig. 2 and Fig. 3).
Females number 1, 8 and 5 produced largest litters, respectively, 10, 11, and 12 kits. The total labour time in these females was similar, respectively, 4 hours and 21 minutes, 3 hours and 42 minutes and 4 hours and 32 minutes. Birth intervals varying between the females are presented in \hyperref[fig1]{Figure 1}. The highest level of activity was found in the female number 1, which left the nest 18 times, whereas the females number 8 and 5 showed a lower level of restlessness (Fig. 2).
The longest total time spent outside the nest was observed in the female number 1, about 43 minutes, whereas in the females number 8 and 5, respectively, 18 and 14 minutes (Fig. 3).
Malmkvist and Palme [2008Malmkvist, J., Palme, R. (2008). Periparturient nest building, Implications for parturition, kit survival, maternal stress and behaviour in farmed mink (Mustela vison). Appl. Anim. Behav. Sci., 114 (1-2), 270–283. https://doi.org/10.1016/j.applanim.2008.01.018.] report that the average parturition in mink takes approximately 6 hours ±28.3 minutes. Houbak and Malmkvist [2008Houbak, B., Malmkvist, J. (2008). Observations of deliveries in mink; potential for more kits. Int. Sci. Conf. “Fur Animal Production” Halifax, 19–23 August 2008, 34–35.], who carried out similar observations, have found that offspring mortality rates were higher in females with a longer total parturition time (2 hours and 10 minutes to 10 hours and 10 minutes) compared with females exhibiting low mortality of kits (1 hour and 2 minutes to 5 hours and 15 minutes). Parturitions of the females analysed in this study were definitely shorter, ranging from 1 hour and 3 minutes to 4 hours and 32 minutes, with an average of 3 hours and 23 minutes.
Braastad [1993Braastad, B.O. (1993). Periparturient behavior of successfully reproducing farmed silver-fox vixens. Appl. Anim. Behav. Sci., 37, 125–138. https://doi.org/10.1016/0168-1591(93)90105-x.], who analysed labour behaviour and parturitions in farmed foxes, demonstrated that with an increasing litter size, parturition duration increased too, whereas birth intervals between kits were shorter. The analysis on mink reveals a similar pattern. The shortest parturition (1 hour and 3 minutes) was observed in the female that produced the smallest litter, and the longest (4 hours and 32 minutes) in that with the largest litter of kits. The periparturient period is a difficult time for the animal, bringing much stress. The natural periparturient behaviour in captivity is even more difficult and heavily depends on the farm conditions. It has not been thoroughly studied how a mink female behave during the parturition on the farm, as is evidenced by rather poor selection of available literature sources. The observations we carried our revealed in the females behaviours showing both serenity and anxiety.
The females number 1, 2, 5, 7 and 8 were quiet in labour, whereas number 4 and 6 were restless. The calm females spent more time nursing their offspring. Before giving birth to other kits, they licked their genital areas. Immediately on the birth, they removed foetal membranes from each kit and ate the placenta. They kept the kits close to their bodies and, catching with teeth, did not allow kits to move too far away. These females spent most time with their young. Differences in the number of departures from the nest were observed between the females. The female number 3, although not so restless as females number 4 and 6, showed a higher level of mobility during parturition, often changed position in the nest, dug through the bedding without much caring about the young born so far. This particular females also showed signs of intensive bleeding from the birth canal.
The females number 4 and 6 exhibited behaviour during parturition that revealed anxiety. Some of the kits were born in the running part of the cage and the dams carried them to the nest. They spent less time licking the newborn kits, often not paying much attention on them, treading on and squashing them. These females anxiously licked their genital areas and nervously pulled out the kits with their teeth.
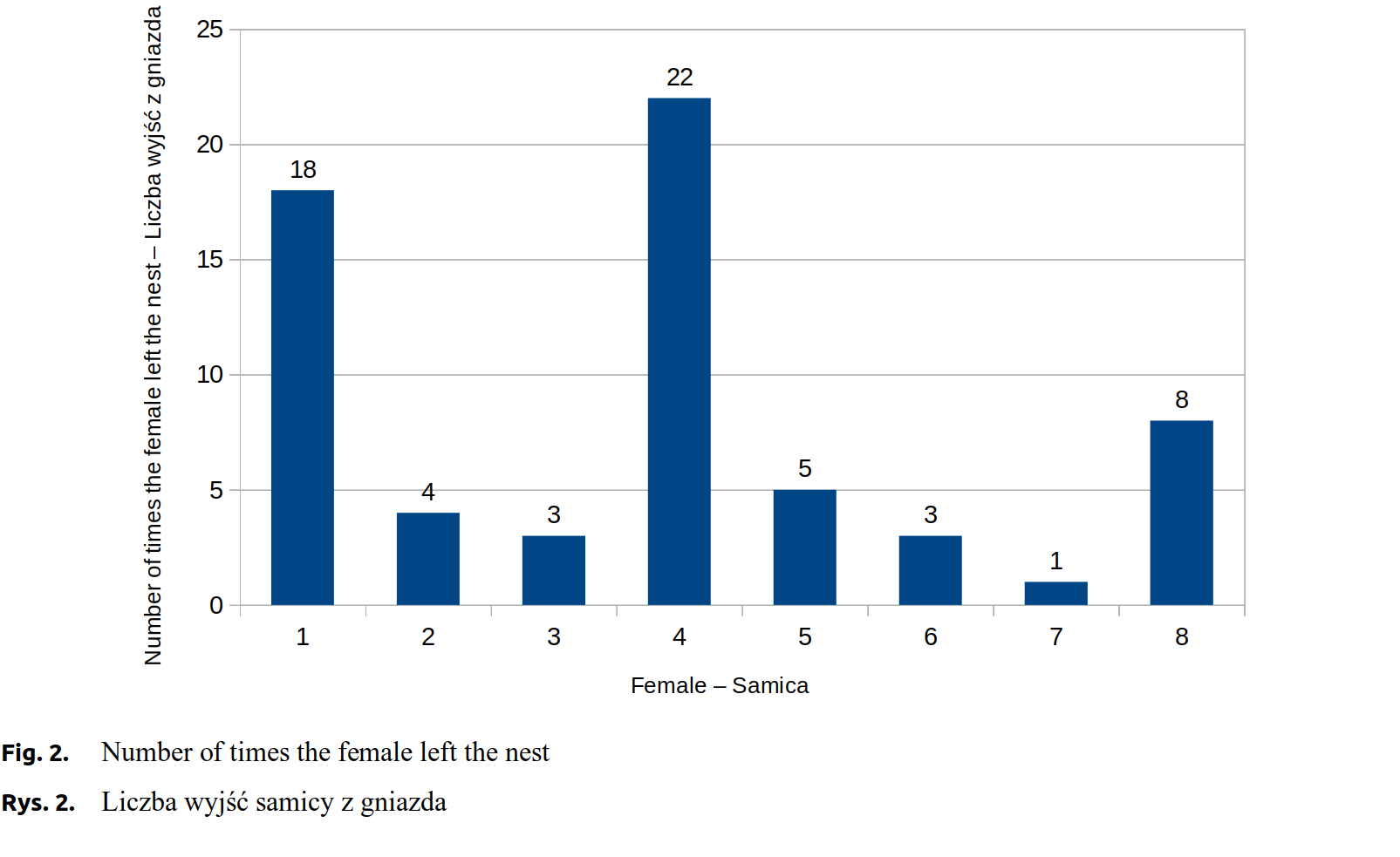
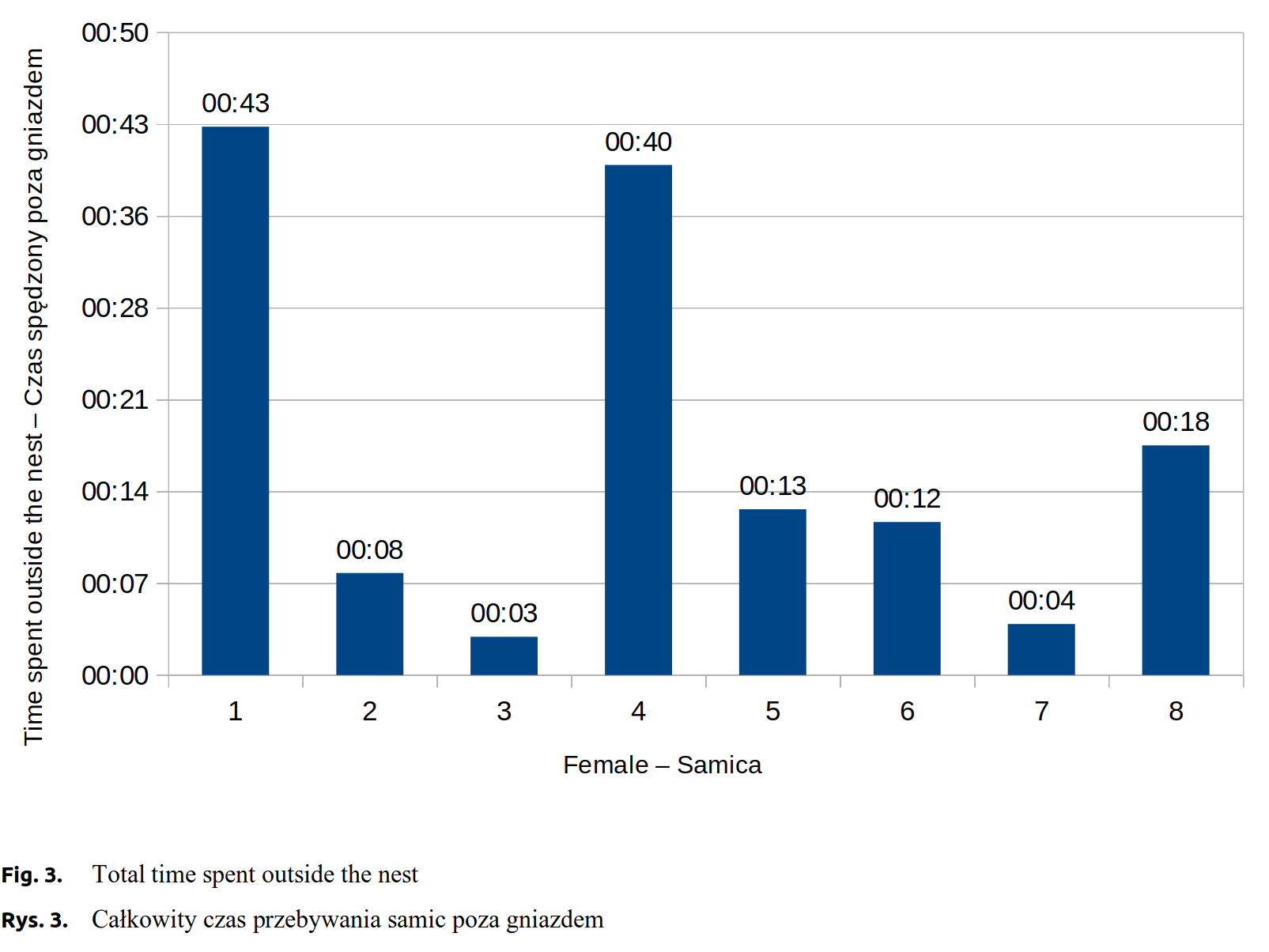
Malmkvist et al. [2007Malmkvist, J., Gade, M., Damm, B.I. (2007). Parturient behaviour in farmed mink (Mustela vison) in relation to early kit mortality. Appl. Anim. Behav. Sci., 107, 120–132. https://doi.org/10.1016/j.applanim.2006.09.018.] carried out studies on differences in the behaviour of American mink females characterised by a low and high mortality rate of the young. Their results indicate that all females during parturition spend similar time in the nest. Females of a higher offspring mortality were less interested in their kits and spent more time idle.
Braastad [1993Braastad, B.O. (1993). Periparturient behavior of successfully reproducing farmed silver-fox vixens. Appl. Anim. Behav. Sci., 37, 125–138. https://doi.org/10.1016/0168-1591(93)90105-x.], who studied periparturient behaviour and parturitions in farmed foxes, found that most females were considerably quiet during labour and did not leave the nest in its progress. Only young and inexperienced females were more restless and anxious. Our observations yielded two groups of behaviour pattern, one showing serenity and the other revealing anxiety. Most females were quiet: thoroughly cleaned their kits from foetal membranes and spent more time on the care. Restless females, on the other hand, left the nest more often and more frequently gave birth outside the nest, showed also less interest in the kits, which were sometimes downtrodden and squashed.
The analysis has not revealed a dependency between total parturition duration and the size of the litter. For the females bearing more kits, after giving birth to the fourth kit, the intervals between subsequent births were longer. The females exhibited varied frequency of absence from the nest during parturition, no relationship has been found between this parameter and the litter size or labour duration. Two behavioural patterns were observed, quiet and anxious; the latter showed less attention in relation to their kits and often gave birth to their offspring outside the nest.
Received: 8 Mar 2019
Accepted: 30 Mar 2019
Published online: 16 Jul 2019
Accesses: 770
Seremak, B., Felska-Błaszczyk, L., Rubinowska, D., Wojciechowska, A., (2019). Analysis of parturitions in Pearl American mink (Neovison Vison). Acta Sci. Pol. Zootechnica, 18(1), 27–32. DOI: 10.21005/asp.2019.18.1.04.