Research Article
Urszula Kaczor1 , Maria Płotkowska1, Elżbieta Martyniuk2
1Department of Animal Biotechnology, University of Agriculture in Krakow, Redzina 1b, 30-248 Cracow, Poland
2Department of Genetics and Animal Breeding, Warsaw University of Life Sciences, Ciszewkiego 8, 02-786 Warsaw, Poland
Abstract. The aim of the study was to identify a potential occurrance of c.*1232G>A polymorphism in the 3'-UTR region of the myostatin gene (MSTN) in sheep of meat breeds: Pomeranian sheep, Suffolk and Berrichon du Cher. The populations of Suffolk and Berrichon du Cher breeds turned out to be monomorphic, whereas in the native Pomeranian sheep, the occurrence of polymorphism in the region of the MSTN gene was demonstrated. The Pomeranian sheep were characterized by a higher frequency of the mutated A allele (0.41), the frequency of genotypes AA and GA was 0.18 and 0.46 respectively. The effect of polymorphism c.*1232G>A on the body weight of ewes on the day of license has not been observed.
Keywords: MSTN gene, polymorphism, body weight, sheep
Myostatin is encoded by the MSTN gene [Boman and Våge 2009Boman, I.A., Våge, D.I. (2009). An insertion in the coding region of the myostatin (MSTN) gene affects carcass conformation and fatness in the Norwegian Spælsau (Ovis aries). BMC Res. Notes, 2, 98. https://doi.org/10.1111/j.1365-2052.2009.01855.x.], growth differentiation factor 8 (GDF-8), which belongs to the family of transforming growth factors-β. After completion of the maturation process, myostatin acts as a negative regulator of skeletal muscle growth in transverse striated muscle [Stefaniuk et al. 2014Stefaniuk, M., Kaczor, U., Kulisa, M. (2014). MSTN gene polymorphism in livestock animals. Post. Hig. Med. Dośw., 68, 633–639. https://doi.org/10.5604/17322693.1103271.]. The decrease in myostatin level as well as the inhibition of its activity significantly increases body weight and accelerates growth of muscle tissue [Dominique and Gérard 2006Dominique, J.E., Gérard, C. (2006). Myostatin regulation of muszle development: Molecular basis, natura mutations, physiopathological aspects. Exp Cell Res., 312, 2401–2414. https://doi.org/10.1016/j.yexcr.2006.07.010.]. It can also cause a decrease in adipose tissue content and increase density of bone tissue [Lin et al. 2002Lin, J., Arnold, H.B., Della-Fera, M.A., Azain, M.J., Hartzell, D.L., Baile, C.A. (2002). Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem. Biophys. Res. Commun., 291, 701–706. https://doi.org/10.1006/bbrc.2002.6500.].
The first research on MSTN gene in livestock was published as early as 1997, just after the studies describing this phenomenon in mice [Grobet et al. 1997Grobet, L., Martin, L.J., Poncelet, D., Pirottin, D., Brouwers, B., Riquet, J., Schoeberlein, A., Dunner, S., Ménissier, F., Massabanda, J., Fries, R., Hanset, R., Georges, M. (1997). A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet., 17(1), 71–74. https://doi.org/10.1038/ng0997-71., McPherron et al. 1997McPherron, A.C., Lawler, A.M., Lee, S.J. (1997). Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90. https://doi.org/10.1038/387083a0.]. Polymorphisms were originally described in two breeds of beef cattle: Piemontese and Belgian Blue. The research showed homozygosity of the Belgian Blue cattle population regarding deletion of 11 base pairs in the coding region nt821 (del 11). In Piemotese cattle, a polymorphism in the myostatin gene was also detected, but in this breed it led to the G-A transition, which caused the replacement of tyrosine with cysteine [Kambadur et al. 1997Kambadur, R., Sharma, M., Smith, T.P., Bass, J.J. (1997). Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res., 7(9), 910–916. https://doi.org/10.1101/gr.7.9.910.% Szukaj .]. Mutations were also identified in other cattle breeds such as Asturiana [Grobet et al. 1997Grobet, L., Martin, L.J., Poncelet, D., Pirottin, D., Brouwers, B., Riquet, J., Schoeberlein, A., Dunner, S., Ménissier, F., Massabanda, J., Fries, R., Hanset, R., Georges, M. (1997). A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet., 17(1), 71–74. https://doi.org/10.1038/ng0997-71.], Maine-Anjou and Charolaise [Grobet et al. 1998Grobet, L., Poncelet, D., Royo, L.J., Brouwers, B., Pirottin, D., Michaux, C., Ménissier, F., Zanotti, M., Dunner, S., Georges, M. (1998). Molecular definition of an allelic series of mutations disrupting the myostatin function and causing double-muscling in cattle. Mamm. Genome., 9(3), 210–213. https://doi.org/10.1007/s003359900727.], Aubrac, Limousine and Pirenaica [Dunner et al. 2003Dunner, S., Miranda, M.E., Amigues, Y., Cañón, J., Georges, M., Hanset, R., Williams, J., Ménissier, F. (2003). Haplotype diversity of the myostatin gene among beef cattle breeds. Genet. Sel. Evol., 35(1), 103–118. https://doi.org/10.1051/gse:2002038.] as well as in Aberdeen Angus and its crosses [Gill et al. 2009Gill, J.L., Bishop, S.C., McCorquodale, C., Williams, J.L., Wiener, P. (2009). Associations between the 11-bp deletion in the myostatin gene and carcass quality in Angus-sired cattle. Anim. Genet., 40(1), 97–100. https://doi.org/10.1111/j.1365-2052.2008.01790.x.]. Polymorphisms in the myostatin gene has also been described in domestic pigeons, goats, pigs, chickens, rabbits, horses [Baron et al. 2002Baron, E.E., Wenceslau, A.A., Alvares, L.E., Nones, K., Ruy, D. C., Schmidt, G. S., Zanella, E.L., Coutinho, L.L., Ledur, M.C. (2002). High level of polymorphism in the myostatin chicken gene. 7th World Congress on Genetics Applied to Livestock Production, August 19–23, 2002, Montpellier, France, 19–23., Stinckens et al. 2008Stinckens, A., Luyten, T., Bijttebier, J., Van den Maagdenberg, K., Dieltiens, D., Janssens, S., De Smet, S., Georges, M., Buys, N. (2008). Characterization of the complete porcine MSTN gene and expression levels in pig breeds differing in muscularity. Anim. Genet., 39(6), 586–596. https://doi.org/10.1111/j.1365-2052.2008.01774.x., Hill et al. 2010Hill, E.W., Gu, J., Eivers, S.S., Fonseca, R.G., McGivney, B.A., Govindarajan, P., Orr, N., Katz, L.M., MacHugh, D.E. (2010). A sequence polymorphism in MSTN predicts sprinting ability and racing stamina in thoroughbred horses. PLoS One., 5(1), e8645. https://doi.org/10.1371/journal.pone.0008645., Zhang et al. 2012Zhang, C., Liu, Y., Xu, D., Wen, Q., Li, X., Zhang, W., Yang, L. (2012). Polymorphisms of myostatin gene (MSTN) in four goat breeds and their effects on Boer goat growth performance. Mol. Biol. Rep., 39(3), 3081–3087. https://doi.org/10.1007/s11033-011-1071-0., Dybus et al. 2013Dybus, A., Proskura, W.S., Sadkowski, S., Pawlina, E. (2013). A single nucleotide polymorphism in exon 3 of the myostatin gene in different breeds of domestic pigeon (Columba livia var. domestica). Vet. Med., 58, 32–38. https://doi.org/10.17221/6654-vetmed., El-Sabrout and Aggag 2017El-Sabrout, K., Aggag, S. (2017). Association of Melanocortin (MC4R) and Myostatin (MSTN) genes with carcass quality in rabbit. Meat Sci., 137, 67–70. https://doi.org/10.1016/j.meatsci.2017.11.008.].
The gene responsible for coding of GDF-8 in sheep is located on the second chromosome. The c.*1232G>A polymorhism originally detected in Texel sheep is characterized by the guanine to adenine transition in the 3′–UTR region. The effect of this mutation is inhibition of myostatin gene translation, i.e. blocking the polypeptide protein synthesis on the mRNA matrix [Clop et al. 2006Clop, A., Marcq, F., Takeda, H., Pirottin, D., Tordoir, X., Bibé, B., Bouix, J., Caiment, F., Elsen, J.M., Eychenne, F., Larzul, C., Laville, E., Meish, F., Milenkovic, D., Tobin, J., Charlier, C., Georges, M. (2006). A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nature Genet., 38(7), 813–818. https://doi.org/10.1038/ng1810.]. Zhang and co wolkers confirm that miR-27b could promote sheep skeletal muscle satellite cell proliferation by targeting MSTN and suppressing its expression [Zhang et al. 2018Zhang, W., Wang, S.Y., Deng, S.Y., Gao, L., Yang, L.W., Liu, X.N., Shi, G.Q., (2018). MiR-27b promotes sheep skeletal muscle satellite cell proliferation by targeting myostatin gene. J. Genet., 97(5), 1107–1117. https://doi.org/10.1007/s12041-018-0998-5.]. The c.*1232G>A poplymorphism leads to reduction of myostatin circulating in the bloodstream by two thirds in the carriers in comparison to individuals with fully functional copies of the MSTN gene [Boman et al. 2010Boman, I.A., Klemetsdal, G., Nafstad, O., Blichfeldt, T., Våge, D.I. (2010). Impact of two myostatin (MSTN) mutations on weight gain and lamb carcass classification in Norwegian White Sheep (Ovis aries). Genet. Sel. Evol., 42(1), 4. https://doi.org/10.1186/1297-9686-42-4.]. Sequencing the open reading frame of the MSTN gene in sheep characterised by increased muscle mass allowed to locate the c.960delG (p.K320NfsX39) in the Norwegian WhiteSheep (NWS) breed [Boman et al. 2009] and c.120insA (p.N40MfsX9) in the Norwegian Spaelsau [Boman and Våge 2009Boman, I.A., Våge, D.I. (2009). An insertion in the coding region of the myostatin (MSTN) gene affects carcass conformation and fatness in the Norwegian Spælsau (Ovis aries). BMC Res. Notes, 2, 98. https://doi.org/10.1111/j.1365-2052.2009.01855.x.].
The aim of the study was to check for the presence of c.*1232G>A polymorphism in the 3′–UTR region of the myostatin gene in meat sheep breeds: Pomeranian sheep, Suffolk and Berrichon du Cher, and if observed, to estimate its influence on the body weight of ewes on the day of getting breeding license.
The study of polymorphism in the gene encoding the myostatin was conducted on a genomic DNA isolated from blood obtained from unrelated ewes of meat sheep breeds: Pomeranian sheep (n = 83), Suffolk (n = 25) and Berrichon du Cher (n = 25). The body weight of ewes was recorded on the day of obtaining a breeding license (over a year old). The local Krakow Ethics Committee for Experiments with Animals approved all experimental procedures relating to the use of live animals.
Blood, from which genomic DNA was subsequently isolated, was collected from the external jugular vein (1 ml) to EDTA tubes. After sampling, it was frozen at –20°C. Genomic DNA isolation was carried out using the commercial reagents kit Sherlock AX (A & A Biotechnology).
For amplification of the MSTN gene, primers of the following sequences were used [Clop et al. 2006Clop, A., Marcq, F., Takeda, H., Pirottin, D., Tordoir, X., Bibé, B., Bouix, J., Caiment, F., Elsen, J.M., Eychenne, F., Larzul, C., Laville, E., Meish, F., Milenkovic, D., Tobin, J., Charlier, C., Georges, M. (2006). A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nature Genet., 38(7), 813–818. https://doi.org/10.1038/ng1810.]:
As a result of the PCR reaction, a 1003 bp fragment was amplified. The reaction was carried out in a 20 μl reaction mixture, 1×Buffer, 2.5 mM MgCl2, 0.2 mM dNTP, 0.2 mM each primer, 0.3 U polymerase (Thermo Scientific) and resulted in obtaining 150–250 ng of genomic DNA. The temperature-time profile of the individual reaction stages was as follows: 3 min – 95°C, 35 cycles: 30' – 95°C, 30' – 59°C, 30' – 72°C; 2 min – 72°C. The digestion of the product was carried out using the HpyCH4V enzyme (New England Biolabs). The visualization of the PCR-RFLP result was performed by electrophoresis in a 2% agarose gel (40 min, 80V, Wide Mini-Sub® Cell GT, BIO-RAD).
The influence of MSTN polymorphism on the body weight on the day when ewes obtaining a breeding license (over a year old) was examined. The normality of the trait distribution was analyzed by the Shapiro-Wilk test. The Kruskal-Wallis test was used to determine the effect of the genotype on the body weight of ewes. A Hardy-Weinberg equilibrium of MSTN genotypic frequencies was also assessed with the χ2 test. The statistical analyses were conducted using the Origin software (OriginLab, Northampton, MA).
Identification of the transition – polymorphism c.*1232G>A in the 3′–UTR region of the myostatin gene in meat sheep breeds: Pomeranian sheep, Suffolk and Berrichon du Cher showed the presence of three genotypes: GG, GA and AA only in case of the Pomeranian sheep. The Suffolk and Berrichon du Cher sheep were monomorphic, in all ewes only the wild-type GG genotype was observed. The Pomeranian sheep were characterized by a higher frequency of the mutated A allele (0.41), and the frequency of AA and GA genotypes was 0.18 and 0.46 respectively (Table 1).
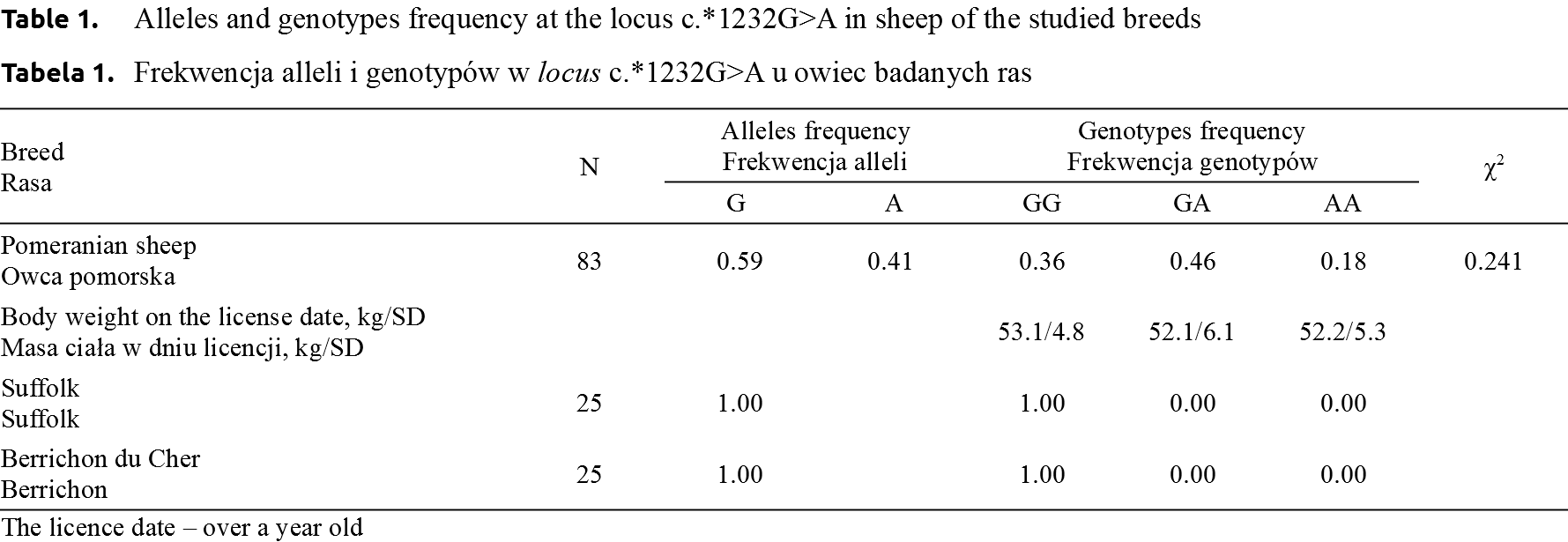
The analyzed population of the Pomeranian sheep was in the Hardy-Weinberg equilibrium as regards the MSTN locus (χ2 = 0.241).
The polymorphism occurring at the site of the MSTN did not have any impact on the body weight of ewes recorded on the day of the license. Mean body weight for genotypes GG, GA and AA was 53.1 kg, 52.05 kg and 52.2 kg, respectively (Table 1).
Polymorphisms in the myostatin gene in sheep are associated with increased muscularity and a decrease in body fat. However, the effects caused by the loss of myostatin function are not as drastic as changes occurring in some cattle breeds [Kijas et al. 2007Kijas, J.W., McCulloch, R., Edwards, J.E., Oddy, V.H., Lee, S.H., van der Werf, J. (2007). Evidence for multiple alleles effecting muscling and fatness at the ovine GDF8 locus. BMC Genet., 8, 80. https://doi.org/10.1186/1471-2156-8-80.]. The high polymorphism of the MSTN gene is proved by 28 polymorphisms that have already been described in sheep [Stefaniuk et al. 2014Stefaniuk, M., Kaczor, U., Kulisa, M. (2014). MSTN gene polymorphism in livestock animals. Post. Hig. Med. Dośw., 68, 633–639. https://doi.org/10.5604/17322693.1103271.].
In the present study, the polymorphism c.*1232G>A (g.6223G>A) in the Pomeranian sheep was identified. The study also shown that Suffolk and Berrichon du Cher sheep populations were monomorphic. The obtained results are similar to Kolenda’s studies carried out in the population of Pomeranian sheep [Kolenda et al. 2019Kolenda, M., Grochowska, E., Milewski, S., Mroczkowski, S. (2019). The associacion between the polymorphism in the myostatin gene and growth traits in Kamieniec and Pomeranian sheep breeds. Small Rumin. Res., 177, 29–35. https://doi.org/10.1016/j.smallrumres.2019.06.007.]. The heterozygous AG carriers were most frequently observed, and the populations were characterized by high variability within the site of the c.*1232G>A mutation. The wild homozygous ewes (GG genotype) in the present study constituted 36% of the analyzed population, which was similar to 38% obtained by Kolenda [2019Kolenda, M., Grochowska, E., Milewski, S., Mroczkowski, S. (2019). The associacion between the polymorphism in the myostatin gene and growth traits in Kamieniec and Pomeranian sheep breeds. Small Rumin. Res., 177, 29–35. https://doi.org/10.1016/j.smallrumres.2019.06.007.]. The lowest frequency in the present study was observed for AA homozygote, only 18%. Nevertheless, it was three times higher in comparison with the analysis by Kolenda et al. [2019Kolenda, M., Grochowska, E., Milewski, S., Mroczkowski, S. (2019). The associacion between the polymorphism in the myostatin gene and growth traits in Kamieniec and Pomeranian sheep breeds. Small Rumin. Res., 177, 29–35. https://doi.org/10.1016/j.smallrumres.2019.06.007.], where frequency of AA genotype was only 6%. Both in the present study and in the one carried out by Kolenda et al. [2019Kolenda, M., Grochowska, E., Milewski, S., Mroczkowski, S. (2019). The associacion between the polymorphism in the myostatin gene and growth traits in Kamieniec and Pomeranian sheep breeds. Small Rumin. Res., 177, 29–35. https://doi.org/10.1016/j.smallrumres.2019.06.007.], the effect of mutations on the body weight of ewes was not observed. The high frequency of heterozygotes observed in Pomeranian ewes included in both studies may suggest that it will be useful to extend research on polymorphism c.*1232G>A and cover a larger part of population of Pomeranian sheep. It would be interesting to analyse the impact of this popymorphism on body weight of lambs during the rearing period. In the future, this trait may be used for selection works with this breed.
The Pomeranian sheep in the current study were characterized by a higher frequency of the mutated A allele (0.41). The c.*1232G>A polymorphism was also detected in different breeds of sheep kept in Great Britain, with a significant predominance of the transitional allele frequency [Hadjipavlou et al. 2008Hadjipavlou, G., Matika, O., Clop, A., Bishop, S.C. (2008). Two single nucleotide polymorphisms in the myostatin (GDF8) gene have significant association with muscle depth of commercial Charollais sheep. Anim. Genet., 39(4), 346–353. https://doi.org/10.1111/j.1365-2052.2008.01734.x.]. The c.*1232G>A mutation is also present in the British Charolais sheep population. However, a higher frequency of wild-type allele was present in this breed. The study suggested possibility to use the c.*1232G>A mutation in breeding programme for Charolais sheep and selection of individuals carrying two copies of the mutant gene, due to their significant increase of skeletal muscles [Hadjipavlou et al. 2008Hadjipavlou, G., Matika, O., Clop, A., Bishop, S.C. (2008). Two single nucleotide polymorphisms in the myostatin (GDF8) gene have significant association with muscle depth of commercial Charollais sheep. Anim. Genet., 39(4), 346–353. https://doi.org/10.1111/j.1365-2052.2008.01734.x.].
Research on the MSTN gene is widely carried out in sheep breeds kept in New Zealand, where c*1232G>A polymorphism was detected in the following breeds: Australian White Suffolk, Dorset Poll, Lincoln [Kijas et al. 2007Kijas, J.W., McCulloch, R., Edwards, J.E., Oddy, V.H., Lee, S.H., van der Werf, J. (2007). Evidence for multiple alleles effecting muscling and fatness at the ovine GDF8 locus. BMC Genet., 8, 80. https://doi.org/10.1186/1471-2156-8-80.] and in New Zealand Romney [Hickford et al. 2010Hickford, J.G., Forrest, R.H., Zhou, H., Fang, Q., Han, J., Frampton, C.M., Horrell, A.L. (2010). Polymorphisms in the ovine myostatin gene (MSTN) and their association with growth and carcass traits in New Zealand Romney sheep. Anim. Genet., 41(1), 64–72. https://doi.org/10.1111/j.1365-2052.2009.01965.x.]. However, these two studies have not demonstrated any effect of any of the MSTN gene variants on traits such as:, birth weight, live weight or growth rate. Another research team found the influence of the MSTN gene on the body weight at birth in Romney lambs; additionally the significant relationship between the genotype in the MSTN gene and the sex of lambs born was observed, where females constituted majority of heterozygous individuals [Han et al. 2010Han, J., Zhou, H., Forrest, R.H., Sedcole, J.R., Frampton, C.M., Hickford, J.G.H. (2010). Effect of Myostatin (MSTN) g.6223G>A on Production and Carcass Traits in New Zealand Romney Sheep. Asian Australas. J. Anim. Sci., 23(7), 863–866. https://doi.org/10.5713/ajas.2010.90392.].
The positive effect on muscularity of one genotype of myostatin was observed in the New Zeland Texel. Individuals characterized by one or two copies of "A" allele were characterised by greater musculature and less fat content in the carcass [Johnson et al. 2009Johnson, P.L., Dodds, K.G., Bain, W.E., Greer, G.J., McLean, N.J., McLaren, R.J., Galloway, S.M., van Stijn, T.C., McEwan, J.C. (2009). Investigations into the GDF8 g.6723G-A polymorphism in New Zealand Texel sheep. J. Anim. Sci., 87(6), 1856–1864. https://doi.org/10.2527/jas.2008-1508.]. Studies on the effect of the c.*1232G>A mutation on adipose tissue in heterozygous crossbred lambs (Scotish Mule ewes by Texel rams) with one copy of an inactive gene, showed a decrease in their fat mass [Masri et al. 2011Masri, A.Y., Lambe, N.R., Macfarlane, J.M., Brotherstone, S., Haresign, W., Bünger, L. (2011). Evaluating the effects of a single copy of a mutation in the myostatin gene (c.*1232G>A) on carcass traits in crossbred lambs. Meat Sci., 87(4), 412–418. https://doi.org/10.1016/j.meatsci.2010.11.019.].
Polymorphisms in the MSTN have also been the subject of research in native Russian sheep breeds. In the Dzhalginsky Merinobreed, twenty SNPs were found, of which three SNPs had a negative effect on the performance, causing, among other things, a decrease in body weight and a daily growth rate. Another three SNPs, among them the c.*1232G>A mutation, had no significant effect on the traits [Trukhachev et al. 2015Trukhachev, V.I., Belyaev, V., Kvochko, A., Kulichenko, A.N., Kovalev, D., Pisarenko, S.V., Volynkina, A.S., Selionova, M.I., Aybazov, M., Shumaenko, S., Omarov, A., Mamontova, T.V., Golovanova, N., Yatsyk, O., Krivoruchko, A.J. (2015). Myostatin gene (MSTN) polymorphism with a negative effect on meat productivity in Dzhalginsky Merino sheep breed. J. Bio Sci. Biotech., 4(2), 191–199.]. Twenty-one SNPs and single mutations consisting of insertions and deletions were identified in the Russian Stavropol Merinosheep. Eight of the observed in the study mutations in the MSTN gene have been described for the first time and identified as unique for this sheep breed [Trukhachev et al. 2018Trukhachev, V., Yatsyk, O., Telegina, E., Krivoruchko, A., Zhou, H., Hickford, J.G.H. (2018). Comparison of the Myostatin (MSTN) gene in Russian Stavropol Merino sheep and New Zealand Merino sheep. Small Rumin. Res., 160, 103–106. https://doi.org/10.1016/j.smallrumres.2018.01.005.].
Research on the polymorphism in the MSTN gene in native Polish sheep breeds was carried out so far only by Grochowska et al. [2019Grochowska, E., Borys, B., Lisiak, D., Mroczkowski, S. (2019). Genotypic and allelic effects of the myostatin gene (MSTN) on carcass, meat quality, and biometric traits in Colored Polish Merino sheep. Meat Sci., 151, 4–17. https://doi.org/10.1016/j.meatsci.2018.12.010.] and Kolenda et al. [2019Kolenda, M., Grochowska, E., Milewski, S., Mroczkowski, S. (2019). The associacion between the polymorphism in the myostatin gene and growth traits in Kamieniec and Pomeranian sheep breeds. Small Rumin. Res., 177, 29–35. https://doi.org/10.1016/j.smallrumres.2019.06.007.]. Polymorphism c.*1232G>A was identified in Pomeranian and Kamieniecka sheep, while the population of the Colored Merino sheep was monomorphic [Grochowska et al. 2019Grochowska, E., Borys, B., Lisiak, D., Mroczkowski, S. (2019). Genotypic and allelic effects of the myostatin gene (MSTN) on carcass, meat quality, and biometric traits in Colored Polish Merino sheep. Meat Sci., 151, 4–17. https://doi.org/10.1016/j.meatsci.2018.12.010.]. The presence of the A-type allele in the population of Pomeranian and Kamieniecka sheep in the Kolenda’s studies as well as in the population of Pomeranian sheep examined in the current study may result from using Texel rams in development of this breeds in order to improve their meat performance. Further studies covering larger population of Pomeranian sheep are required in order to fully explain the relationship between the polymorphism within the MSTN gene and the traits of meat performance and consider their possible applications.
This study was supported by the University of Agriculture in Krakow, project no. DS/3242.
Received: 15 Apr 2019
Accepted: 20 Jul 2019
Published online: 30 Sep 2019
Accesses: 1082
Kaczor, U., Płotkowska, M., Martyniuk, E., (2019). Identification of c.*1232g>a polymorphism in the MSTN gene in meat sheep breeds in Poland. Acta Sci. Pol. Zootechnica, 18(2), 19–24. DOI: 10.21005/asp.2019.18.2.03.